The Promise of RNA-Based Therapies
Published 11-30-22
Submitted by CRB
Decades of scientific progress in understanding the root cause of many formerly intractable diseases has opened up new treatment options, namely through RNA-based therapies. This second wave of biopharma, in which our understanding of the ways coding portions of our genome relate to disease, allows us to intercede earlier, and at more specific and effective junctures.
For decades, biopharma therapies focused on monoclonal antibodies (mAbs) as tools to trigger the body’s systems to enact desired therapeutic actions. Monoclonals have been revolutionary, bringing significant impacts to millions of patients.
Currently, RNA technologies—like oligonucleotides and mRNA—are in the spotlight as the biopharma industry seeks new tools to meet healthcare demands. The headline-grabbing mRNA vaccines that helped pull us out of the COVID-19 pandemic are just the first glimpse at how transformative this next wave of biopharma can be to the practice of healthcare. RNA therapies allow us to design therapies for diseases we could not treat before and better address other conditions using drugs with improved tolerability, dosing regimens, and clinical efficacy.
Almost all experts we surveyed for our Horizons: Life Sciences report who are involved with RNA technology said their companies will be emphasizing RNA-based therapies in their future pipelines. They believe that these technologies have the potential to replace existing platforms and present opportunities beyond vaccine development.
Here, we delve into a description of RNA-based technologies, their therapeutic applications and challenges, and considerations for manufacturers considering these innovative products.
- mRNA
- siRNA (small interfering RNA)
- miRNA (micro RNA)
- sgRNA (single guide RNA) – While sgRNA is not a therapy in and of itself, it is a component of CRISPR-Cas9 systems, which are therapies that act on DNA
- ASO (antisense oligonucleotides)
- aptamers
What is RNA?
Ribonucleic acid is a set of molecules that hold and express information transcribed from DNA. Most organisms use DNA as their genetic code, though some—notably viruses, like SARS-CoV-2 and flu—have RNA genomes.
There are three kinds of naturally occurring RNA:
- Messenger RNA – Genes are transcribed from DNA into mRNA, then translated into protein.
- Ribosomal RNA – rRNAs are structural and functional parts of ribosomes, the protein factories that translate mRNA into protein.
- Transfer RNA – tRNA is integral to protein production.
RNA-based therapiesA bit of history
RNA was considered as a potential therapeutic as early as 1990 when RNA containing gene sequences was injected into live mice and gene expression was observed. This proof-of-concept experiment was followed by a study in which mRNA injected into mice temporarily reversed a genetic disease via the expression of vasopressin. Even mRNA vaccines—which have been so successful to combat COVID-19—have been around since they were first tested as a means to express tumor-specific antigens three decades ago.
While RNA has many functions in the cell, most of its relevant therapeutic applications involve manipulating the pathway in which DNA is transcribed into mRNA, then translated into protein. RNA technologies are being applied to treat a broad range of conditions, such as infectious diseases, cancer, autoimmune diseases, high cholesterol, and rare diseases.
RNA-based therapies differ from other biologics
RNA drugs require an understanding of protein-encoding genes and how to manipulate the process of protein production that occurs outside the cell nucleus for therapeutic benefit. This can use mRNA, which will be translated into a protein that may be missing within the cell, or siRNA, which binds to and interferes with the translation of endogenous mRNAs.
RNA-based therapies have a range of mechanisms of action.
- mRNA can encode an important protein that is damaged or missing
- siRNA can interfere with protein production by binding to and inactivating mRNA
- Aptamers can bind to and inactivate peptides and proteins
- ASOs can modulate gene expression
- sgRNAs, when used with gene editing enzymes (e.g., in CRISPR-Cas9), can direct the editing of DNA or RNA
RNA therapies tend to be quicker to develop, approve, and see widespread adoption than existing biologics, like monoclonal antibodies, cell and gene therapies, and gene editing techniques like CRISPR.
What are the different RNA platforms?
mRNA
Synthesized mRNA can be introduced into cells where it is translated into an innate or acquired protein by the existing cellular machinery.
Vaccines
mRNAs expressing an antigen have been experimentally introduced into cells in an attempt to vaccinate against viral diseases, like HIV and flu, as well as against cancers, including melanoma. This tactic has been successfully applied in COVID-19 vaccines, in which mRNA is packaged in lipid nanoparticles (LNPs).
Protein replacement therapies
This gene therapy corrects the adverse effects of a missing or defective protein. This can be done by transfecting cells with DNA, upon which it is inserted into the genome and stably expressed, or by adding mRNA to cells, after which a functional protein is transiently expressed.
Companies are developing mRNA replacement therapies for metabolic conditions, cystic fibrosis, heart diseases, and immunomodulators for oncology treatments.
It has also been used, as part of a CRISPR-Cas9 treatment, to knock out the mutant transthyretin gene present in patients with transthyretin (ATTR) amyloidosis.
siRNA
Small interfering RNAs are short (roughly 20 nucleotides) double-stranded oligonucleotides with sequences complementary to the mRNA of an expressed gene. They bind to that mRNA, forming a double-strand RNA molecule that is then targeted by the cell for degradation. This process is known as gene silencing and is a normal part of post-transcriptional regulation of gene expression.
siRNA has been used to treat HIV and cancer. Three siRNA drugs have been approved by the FDA, including Onpattro, Giviaari, and Oxlumo, while at least seven are in phase 3 clinical trials.
miRNA
miRNAs are similar in function to siRNA but are typically shorter chains of nucleotides designed to bind more promiscuously to mRNA; whereas siRNA binds specifically to the mRNA of one gene, miRNA can interact with multiple mRNAs.
ASO
These are short (13–30 nucleotides), synthetic single-strand oligonucleotides that, like siRNA, bind to a specific cellular mRNA, depending on their sequence. This binding alters gene expression through enzyme-mediated degradation of the mRNA or, alternatively, through a process known as alternative exon splicing. ASOs are extensively modified with synthetic nucleosides, rendering them stable and removing the need for a delivery vehicle.
Spinal muscular atrophy is caused by incorrect mRNA splicing of the SMN2 gene in which one of the exons in the pre-mRNA is skipped and not included in the final mRNA. This means an incomplete, and dysfunctional, protein is translated. Spinraza (nusinersen) is an FDA-approved RNA drug that binds to the pre-mRNA and allows it to be processed correctly.
Other FDA-approved examples include milasen, used in a single patient to treat Batten disease; fomivirsen for the treatment of cytomegalovirus retinitis; several exon-skipping oligos for the treatment of Duchenne muscular dystrophy (e.g., eteplirsen, golodirsen); mipomersen for familial hypercholesterolemia; and inotersen for hereditary transthyretin-mediated amyloidosis.
sgRNA
Single guide RNA molecules act to steer enzymes that target DNA in CRISPR gene editing systems. They are typically more than 100 nucleotides in length and consist of two sequences fused to make one sgRNA: crispr or seeking RNA is 17–20 nucleotides long and binds to the target DNA sequence to be edited; tracrRNA or scaffold RNA binds to the DNA editing enzyme.
Aptamers
Aptamers are short (15–45 nucleotides) single-stranded oligonucleotides that have high-affinity binding to cellular targets, such as proteins and viruses. The hope is that they could be used therapeutically to inactivate viruses, for example by binding to the spike protein of the SARS-CoV-2 virus. Pegaptanib (27 nucleotides) is the only FDA-approved RNA aptamer drug and is used to treat macular degeneration.
Other cutting-edge RNA-based therapies
A number of experimental RNA therapies exist that are not yet therapeutically relevant, including self-amplifying RNA (saRNA), circular RNA (cirRNA), and tRNA. There are approximately 80 different types of synthetic and natural RNAs, suggesting there is still plenty of room for experimentation and development.
Synthesis of RNAsChemical synthesis
Generally, shorter RNAs and oligonucleotides (e.g., siRNA) are manufactured via solid phase chemical synthesis in vitro, using modified nucleosides, commonly phosphoramidites. These modifications to an oligonucleotide help prevent degradation, improve binding to RNA or DNA target sequences, and increase stability in the body. The large volumes of organic solvents, expensive raw materials, and the fact that synthetic yield decreases with each additional nucleotide added, mean this is not an ideal process for longer molecules. The controlled nature of the process means it can accept a wide variety of nucleoside and nucleoside-linkage modifications, both of which have shown therapeutic relevance, allowing for precise tailoring of the molecule’s pharmacodynamics.
In vitro transcription (IVT)
Longer RNAs, like mRNA, are produced by IVT, during which nucleotides are assembled into the desired RNA chain from a DNA template via a transcriptase-mediated process. This process occurs in an aqueous environment, has high accuracy, and is comparatively quick, but transcriptase cannot use heavily modified nucleosides and cannot introduce alternate linkage strategies.
Therapeutic applications
The power of RNA therapies is, in part, their ability to regulate protein production in the cell. Proteins are a diverse class of molecules affecting cell structure, transport, catalysis, messaging, and modulating functions in virtually all biological pathways vital to human life. When proteins are absent, overabundant, or non-functional, critical biological processes are affected and can lead to disease.
Proteins are also key to viral and bacterial function, and influence the course of infection. Our bodies’ natural defenses against pathogens often use recognition of, and interference with, these foreign proteins.
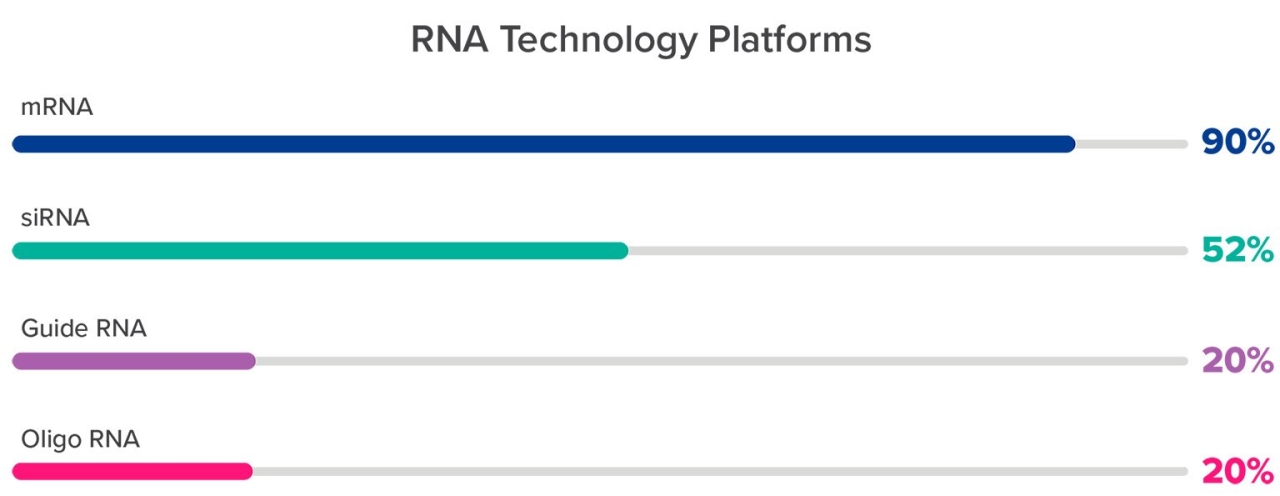
The ubiquitous presence of proteins means modulating them can have broad-ranging therapeutic effects. For this reason, it’s not surprising the average company pursuing RNA-based therapies intends to apply these to multiple indications (Figure 1). The benefit of RNA therapies is that all RNA manufacturing platforms are easily adapted from one protein target to another, because all that changes is the nucleotide sequence.
Cellular protein regulation
Proteins serve a large number of diverse functions in cells, from structural to functional. They are incredibly diverse compounds and many diseases result from a cell producing too much or too little of a specific protein. An RNA therapy, by modifying the production of a specific protein, can lead to an increase or decrease in protein levels and, thus, have an effect on a disease state.
Generally, oligos decrease the amount of protein in a cell by causing the degradation of innate mRNA that would be translated into protein. mRNA therapies increase the amount of a protein in a cell by providing instructions for protein synthesis by the cellular machinery.
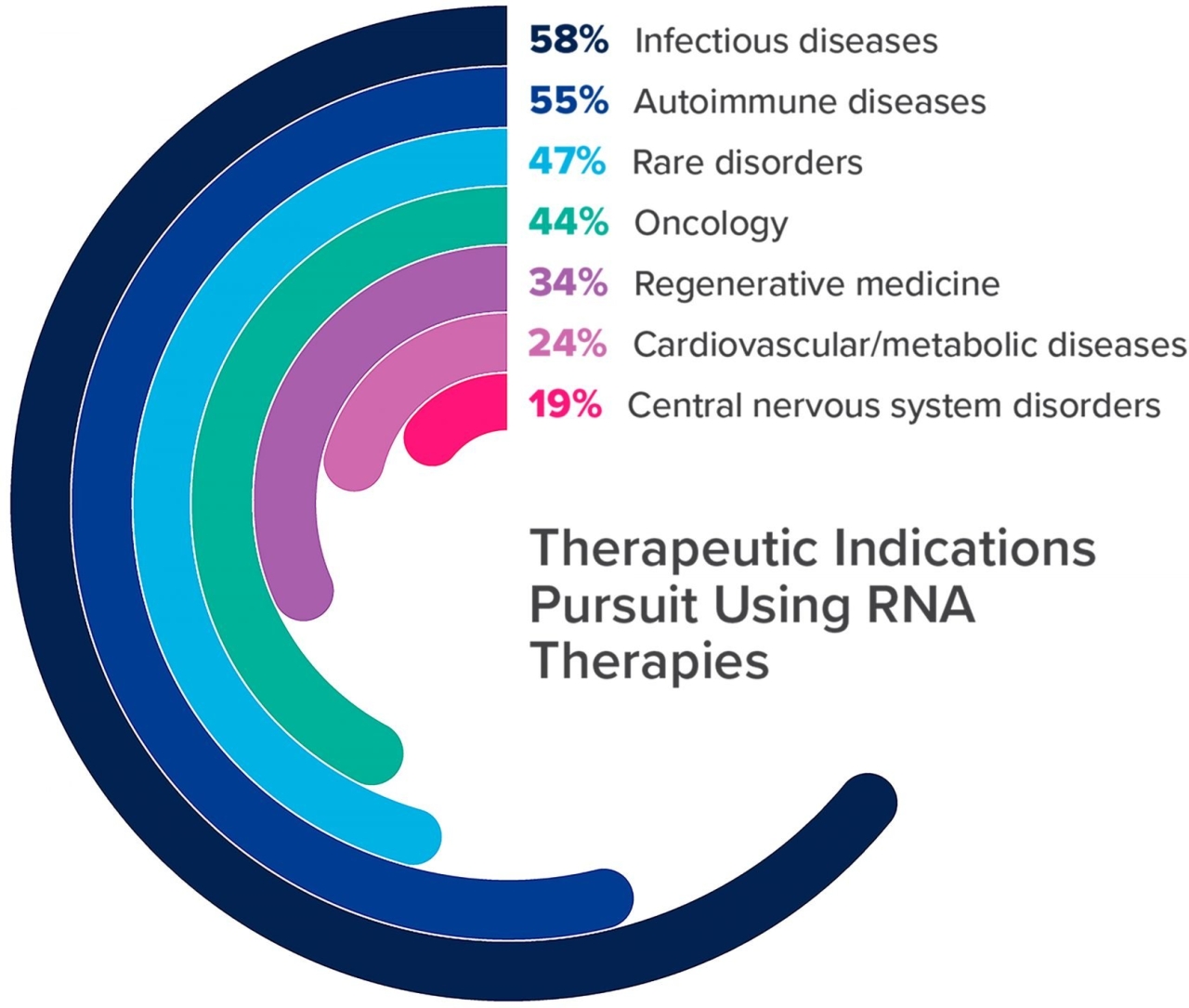
Companies intend to use RNA-based therapies for infectious diseases, autoimmune diseases, rare disorders, oncology, regenerative medicine, cardiovascular and metabolic disorders, and central nervous system disorders (Figure 2).
The push to develop RNA-based therapies for autoimmune disorders (e.g., rheumatoid arthritis and psoriasis) suggests there is a belief that they can improve upon existing effective small molecule (e.g., tofacitinib/Xeljanz) and biologic medical products (e.g., etanercept/Enbrel). This could include improvements in both tolerability and therapeutic benefit as the monoclonal antibody treatments for autoimmune disorders tend to have significant side effects, require large doses, and are expensive to make.
Vaccines
mRNA vaccines have been an excellent entry into the RNA market for many companies. The success of the COVID-19 vaccines convinced many in the industry that mRNA vaccines and oligos belonged in their pipelines—their effectiveness, combined with the relative ease of production, make them difficult to ignore. Flu vaccine manufacturers are considering a switch away from cell-based vaccine production to mRNA to take advantage of substantial potential benefits:
- Faster production
- Ease of switching between mRNA molecules to target emerging seasonal virus strains
- Smaller doses
- Lower cost of goods
The success of COVID-19 vaccines also means a facility geared to produce an mRNA vaccine could be used to produce mRNA therapies for other indications, including autoimmune disorders, immunotherapy, and oncology. In fact, prior to COVID-19, mRNA companies were best known for their work developing cancer vaccines.
Almost all those in our Horizons survey believe RNA-based therapies will have potential beyond mRNA vaccines or will be disruptive in drug manufacturing (Figure 2).
Protein blocking
Aptamers are like chemical antibodies, binding to protein targets with high specificity within a cell and inactivating them. They are much simpler and less expensive to manufacture than monoclonal antibodies, and are more stable in the body. They are being developed to diagnose and treat infectious diseases (e.g., HIV, influenza B, tuberculosis) and various types of cancer.
Examples of ways CRISPR-Cas9 is being tested include to correct a point mutation that causes blindness, allow production of gamma-globin from a developmentally silenced gene, and to create allogeneic CAR-T cells for the treatment of various cancers.
Gene modifications
CRISPR and other gene-editing will be part of the third wave of biopharma. While the technology is advanced enough to provide proof-of-concept experiments, the social and ethical implications of permanently modifying the genome of any organism—let alone humans—need to be resolved before these technologies can be used therapeutically on any but unaddressed and very serious diseases.
Manufacturing RNA-based therapies is faster and cheaper
RNA technologies have the benefit of reducing facility design and construction costs when compared to a conventional biologics facility, largely because there is no need for cell culture. Here are a few other benefits.
Increased speed to market
Development times are greatly reduced, in part because of the ease of switching from one RNA molecule to another. For example, the raw materials, equipment, and process suites remain the same when switching from mRNA for a vaccine to mRNA for oncology. It could require little more than cleaning process equipment between batches, reducing both costs and downtime. This means that multiple RNA molecules can be produced in a facility using the same technology with minimal additional expense.
Lower cost of goods
While the raw materials to produce RNA are expensive—including a high cost per gram for modified nucleosides, specialized enzymes, and plasmid DNA—the overall cost of goods is lower because small amounts of each are needed. As well, increasing demand has led to increased supply and decreased prices over the past two decades.
Utilities costs are lower when compared to making a biologic (mAb), including water for injection (WFI), clean-in-place/steam-in-place (CIP/SIP), and electricity.
Regulatory platforming
The speed with which manufacturers developed, tested, and produced vast quantities of safe and effective mRNA vaccines for COVID-19 was astounding. This proof of concept on a global scale should smooth the regulatory path for other RNA-based therapies.
When it comes to regulations, a rising tide floats all RNA boats.
A strong argument might be made that, when a company wants to modify the mRNA in its COVID-19 vaccine to respond to a new variant, it could shorten safety studies. Since the mRNA would be in the same formulation, all that might be needed is to assess its efficacy.
Challenges of RNA-based therapies
Among the challenges inherent in using RNA as a therapeutic are instability, delivery, and targeting.
Cold supply chain
RNA is an unstable product, needing protection from conditions that favor degradation. One way this is done is to use modified nucleosides to manufacture RNA, stabilizing the molecules during production and in the body. Another way is through cold storage, which was used for the early COVID-19 mRNA vaccines that were stored at ultra-cold temperatures. Subsequent studies showed they could be stored stably in conventional freezers at –20oC, making the supply chain simpler.
Instability in vivo
RNA is inherently unstable in vitro and susceptible to in vivo enzymatic degradation by RNAase as part of the body’s innate immune response. This instability—or short half-life—was first overcome experimentally by making mRNA that contained a modified nucleoside, pseudouridine. Solid phase chemical synthesis increases in vivo stability.
The need for tissue-specific delivery
The inability to target specific tissues with RNA can limit what conditions can be treated. Unless it’s injected into spinal fluid, for example, which is painful, the drugs tend to be filtered through the liver.
Delivery systems
Delivery vehicles are needed to overcome the challenges of RNA instability, cellular uptake of large, negatively charged molecules, and the ability to target specific tissues. While viral delivery has been used successfully, it is limited by the size of the possible RNA payload and the expense of making vectors. Non-viral alternatives that protect RNA and improve cellular uptake are gaining traction.
Encapsulation
Liposomes and micelles
These lipid-based structures form either a lipid monolayer (micelles) or bilayer (liposome) to mimic cell membranes. Liposomes were used to encapsulate and deliver mRNA to cells for the first time in 1978 and delivered an miRNA therapy in a phase 1 trial.
Polymers
Cationic polymers, such as poly-beta-amino-esters (PBAEs), complex with RNA to form nanoparticles. Polymer drug delivery systems have been approved by the FDA for small molecules and are in development and preclinical studies for RNA-based therapies.
Lipid nanoparticles
LNPs are spherical protective vesicles mimicking viral vectors. Early versions consisted solely of cholesterol and phospholipids. Continued development has led to FDA-approved LNPs that consist of four components:
- Ionizable cationic lipids
- Phospholipids
- Cholesterol
- PEGylated lipids
The first FDA-approved use of an LNP to deliver an RNA therapeutic was the siRNA drug, patisiran (Onpattro®) for the treatment of hereditary transthyretin amyloidosis (hATTR). Patisiran is a gene silencing therapy that uses an LNP to target delivery to liver cells where the mRNA inhibits synthesis of transthyretin. LNPs have also been used successfully to deliver mRNA COVID-19 vaccines.
Conjugates
Conjugates are molecules linked to RNA so they target specific tissues. For example, GalNAc (N-acetylgalactosamine) is a carbohydrate that binds to a receptor on hepatic cells. When GalNAc is linked to siRNAs, they are delivered specifically to the liver. GalNAc conjugation also improves siRNA stability in the absence of a delivery vehicle and improves cellular uptake.
Alnylam has three FDA-approved GalNAc-conjugated siRNA drugs, each of which has three GalNAc ligands for more avid binding: givosiran (for acute hepatic porphyrias), inclisiran (hypercholesterolemia), and lumasiran (primary hyperoxaluria type 1).
mRNA opens the door for modular and deployable facilities
mRNA production does not require cell culture, eliminating the need for the kind of huge stainless steel bioreactors and large-scale downstream processing equipment needed for other biologics. This contained production means there is no longer a good argument for the economy of scale of building one facility to produce enough drug product treat the whole world.
Because a much larger number of final doses can be made from a small mRNA synthesis, there is a push for modular facilities that can be erected locally and quickly.
- A relatively small capital investment makes it possible for a country without access to large-scale pharmaceutical manufacturing capacity to build an mRNA facility to provide enough vaccine doses for domestic use.
- Facilities can be built closer to patients.
- The same facility could be used for multiple, different mRNA products simultaneously or sequentially.
- Local production simplifies supply chain logistics and shipping.
Scaling up mRNA production can be as simple as adding an additional module. These are, in fact, being produced by BioNTech in shipping container-sized modules to facilitate shipping around the globe. A complete facility, with office and warehouse modules, can be built and expanded or contracted as needed. While the type of facility will be different for different RNA therapies, within each category (e.g., mRNA, siRNA) the platform for making bulk drug substance is virtually the same.
The ease of switching from one mRNA therapy to another means a country can consider building domestic production facilities. Major biopharma companies are interested in helping nations develop domestic mRNA vaccine manufacturing capacity in places like Brazil, Australia, and Singapore. We could soon see smaller RNA facilities popping up all over the world.
In-house production or CDMO?
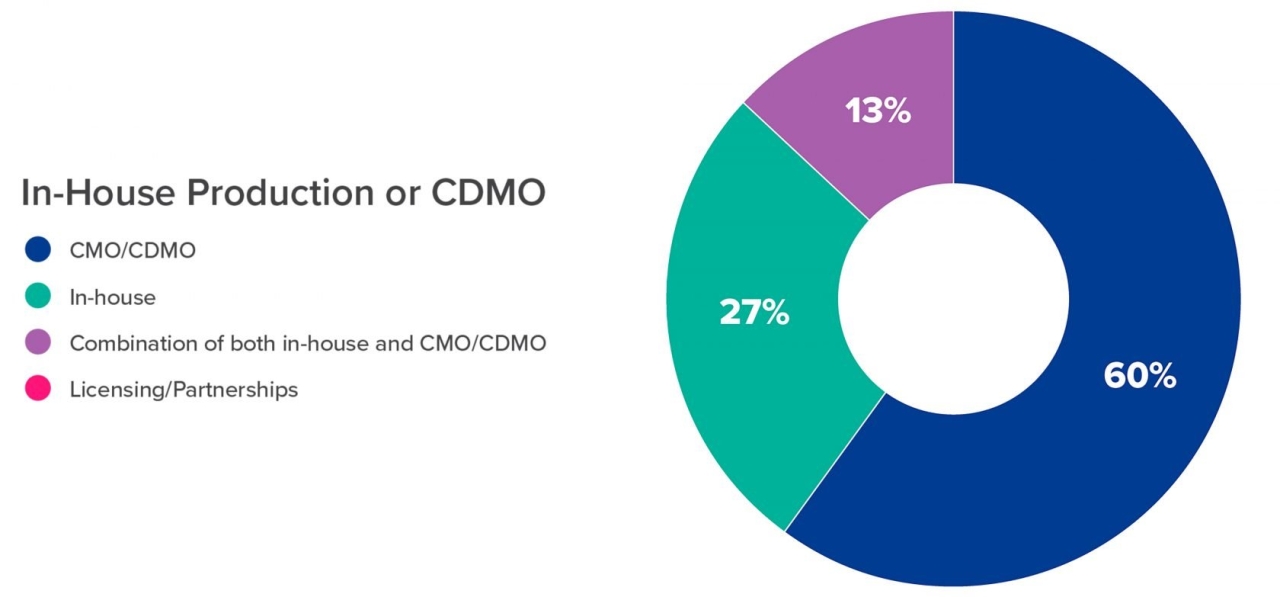
The majority (60%) of those surveyed intend to rely on a CDMO for the production of the relatively small amounts of RNA needed for development and clinical trials (Figure 3). This amounts to microgram to milligram quantities for discovery and development phase production, while clinical trials require gram quantities.
However, large-scale vaccine production, as the type seen for COVID-19, requires kilogram quantities of mRNA per year. When you combine these requirements for large amounts with the lack of available production capacity in the CDMO space—meaning a company could wait two years for its first batch— it is no surprise that many companies are planning in-house manufacturing. The demand for contract manufacturing, coupled with rising prices are suggesting that it makes sense to build in-house capacity to maximize the return on investment.
This requires planning since it can take an average of three years to get a facility up and running. Planning future capacity needs for a drug—then designing a right-sized facility to meet estimated demand—has always been a challenge for manufacturers. Predicting demand and captured market share, in addition to clinical trial success, creates additional business risk when undergoing facility planning. Reducing these risks requires an expert design team capable of allowing for flexible growth.
The future of RNA
RNA-based therapies will provide options for currently unaddressed orphan diseases, as well as improving on drug products already in use for many indications. RNA therapies require different manufacturing facilities than traditional mAb facilities but, in many ways, these facilities have development and manufacturing advantages. RNA-based therapies also have the benefits of shortened development times, reduced facility footprint and capital costs, and rapid adaptability to other indications. This makes it likely that RNA drug products will expand at the expense of the large molecule drugs they will replace due to speed-to-market and ease of regulatory approval.
The thrilling question researchers are asking is, What disease are we going to cure today? With the technology available and in development they have the genetic sequences, can synthesize the RNAs, and have the delivery platforms. They can switch from one product, for one indication, to another product, for another indication in a matter of months. For this wave of RNA-based biopharma, the sky’s the limit.

CRB
CRB
CRB is a leading global provider of sustainable engineering, architecture, construction, and consulting solutions to the life sciences and food and beverage industries. Our innovative ONEsolution™ service provides successful integrated project delivery for clients demanding high-quality solutions -- on time and on budget. Across 21 offices in North America and Europe, the company's nearly 1,800 employees provide world-class, technically preeminent solutions that drive success and positive change for clients and communities. See our work at crbgroup.com, and connect with us on social media here.
More from CRB

